Let’s talk about polymers in ophthalmology. It’s no secret that I’m fascinated by the various applications of polymers. I have also spent the last couple of years developing polymer-based formulations for back of the eye drug delivery for my PhD. Now, I was curious to find out where else polymer materials are used in and around the eye. I started with a quick search that turned into a week of reading, because it seems, polymers and eyes go really well together. To cover all the clinical applications (that I found), I decided to split this post into several parts. Here is part 1 dealing with Viscoelastic & Drug Delivery Formulations.
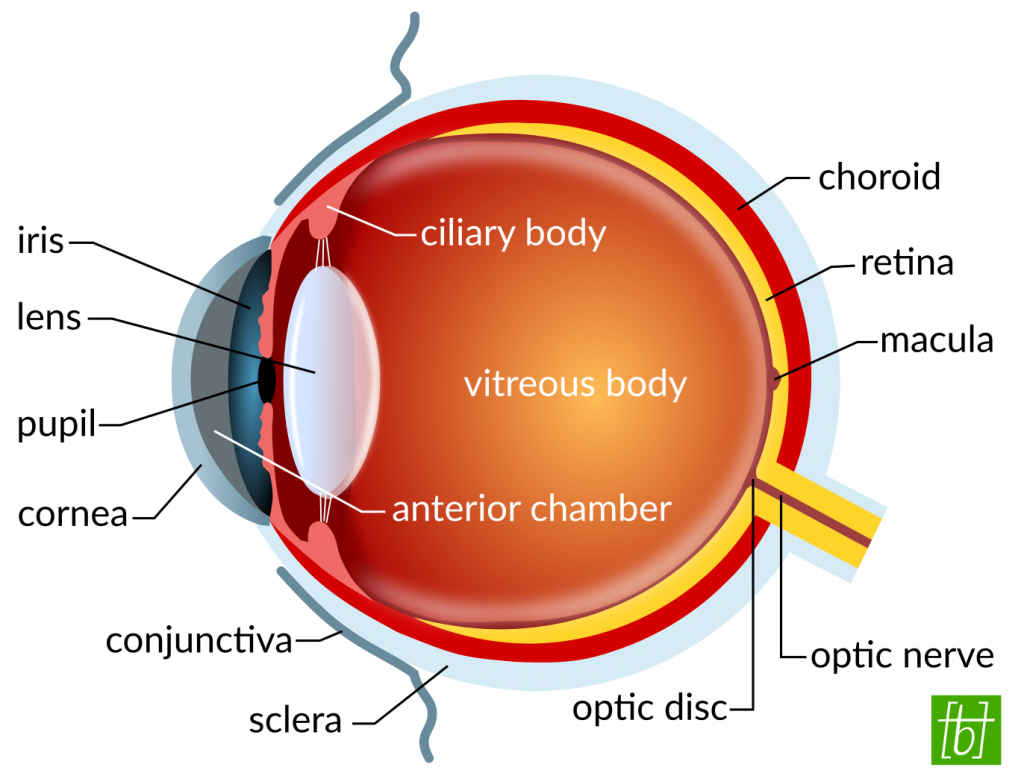
Topical Formulations
The first example that you are probably familiar with are eye drops. Did you know that many eye drop recipes contain polymers to modify their viscosity? Through the addition of macromolecules, the solutions become more viscous. Some contain cross-linked polymer networks that are swollen with water, so called hydrogels. Others contain polymers that only form gels upon contact with the eye surface. A high viscosity helps the drops to stay longer on the cornea and, thus, have a longer lasting moistening effect. Because the main problem of all topical formulations is their short residence time. They are quickly washed away by tear flow and blinking.
If the eye drops contain drugs, like antibiotics or anti-inflammatory substances, a longer residence time benefits drug absorption into the eye. Though, penetration of drugs through the cornea is greatly hindered by the tight junctions between the cells. Therefore, only about 5 % of therapeutics are typically reaching the anterior chamber. Despite the low drug absorption, eye drops are still the formulation of choice, because of their easy use and non-invasive nature. Commonly used polymers in eye drops include:[1]
- cellulose derivatives
- polysaccharides, like sodium hyaluronate or gellan gum
- carbomer, i.e. crosslinked poly(acrylic acid)
- polyvinyl alcohol
- poloxamers, i.e. poly(ethylene oxide)-poly(propylene oxide) block copolymers
Inserts are solid or semi-solid polymer rods or sheets that are inserted under the eyelid or directly placed onto the cornea.[1] They were mainly developed for drug therapy but are rarely used. They are difficult to place correctly by the patient, interfere with vision and can induce foreign-body sensations. There are soluble inserts made of collagen, which disintegrate over time. Other insoluble inserts can contain poly(meth)acrylates, polyvinyl acetates, or cellules derivatives. Those have to be removed after the drugs are depleted.
Drug Delivery Systems
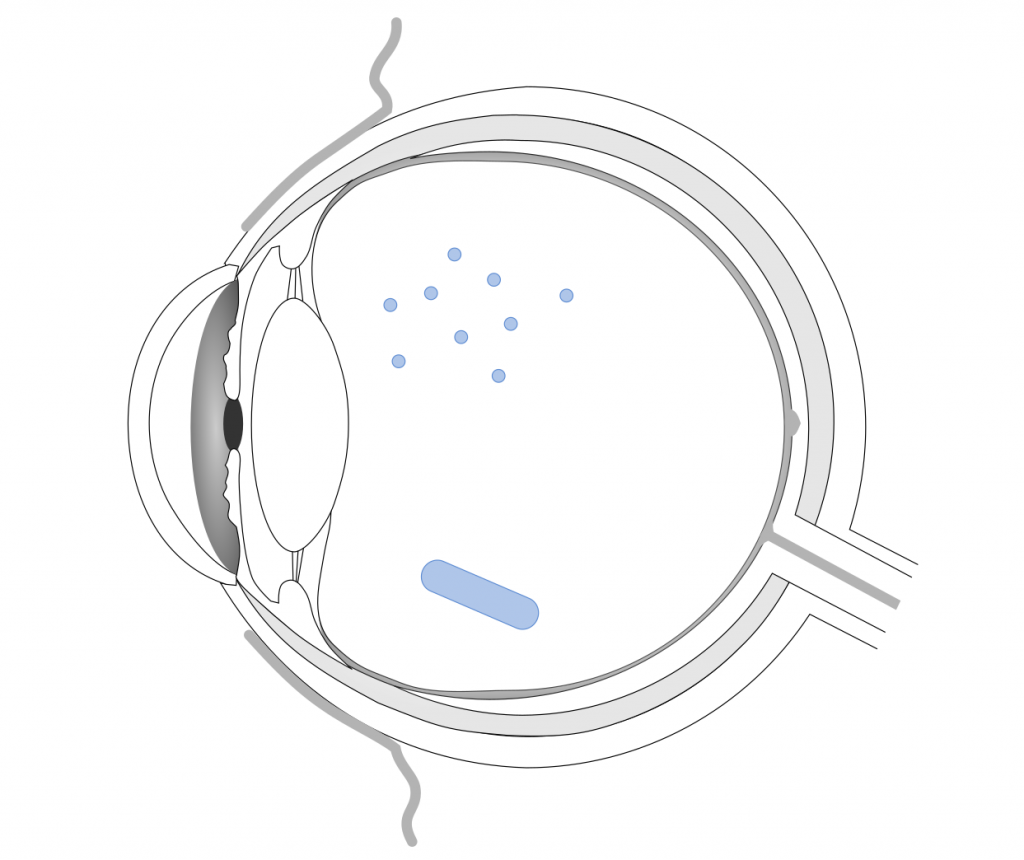
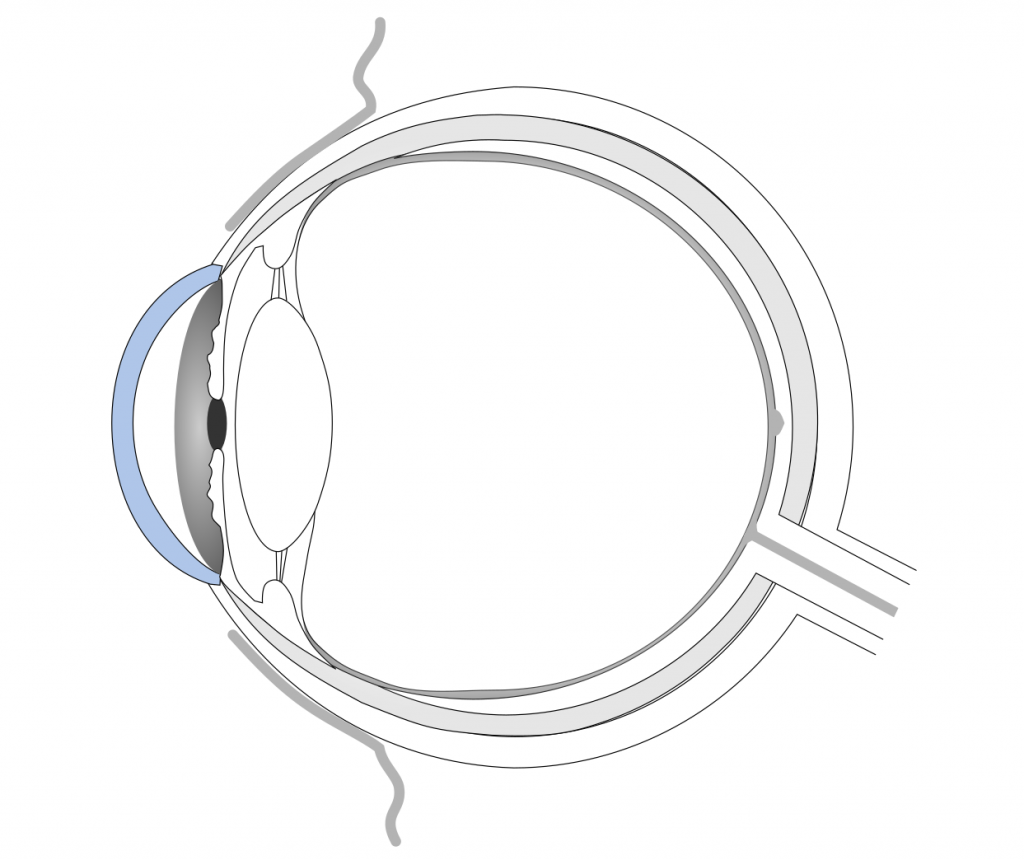
Intraocular drug delivery is an incredibly fascinating and challenging topic. It is challenging because the eye is built to keep out harmful substances, like toxins or microorganisms, and consequently also keeps out drugs. As mentioned above, drugs applied via eye drops hardly reach the back of the eye. Hence, the therapeutics have to be injected of implanted close to the site of desired action. Drug-loaded polymer formulations can sustain the release of therapeutics and decrease the frequency of required injections. They are currently a hot topic in ophthalmic research. Polymer depots can be placed in the anterior chamber, vitreous, or in the periocular space, which is the space surrounding the eyeball. Intraocular drug delivery is required for the treatment of e.g. retinal diseases or uveitis (inflammation of ocular tissues).
Although many types of polymer formulations, such as micro- and nanoparticles, hydrogels, and implants, are investigated, only a few polymer implants are FDA-approved yet.[2] They consist of either biodegradable polyesters, like poly(lactic acid-co-glycolic acid) (PLGA), or non-degradable biocompatible polymers (e.g. polysiloxane, polyvinyl alcohol, polyimide, etc.). Current implants deliver anti-inflammatory corticosteroid drugs. There is still a lot of room for improvement, prolonging the delivery periods, increasing the bioavailability of drugs, and reducing side effects (visual disturbance, development of cataract, increased intraocular pressure). This might be a topic for another post soon.
Viscoelastic Fluids
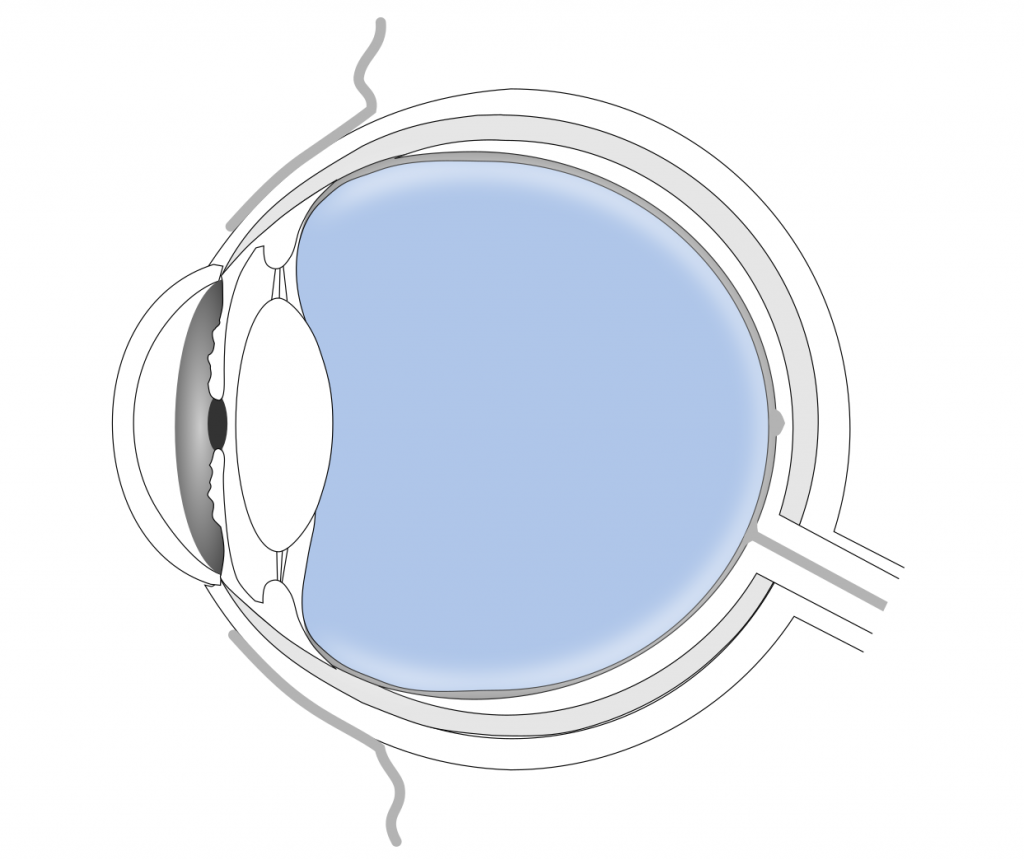
Vitreous substitutes are substances used to replace the vitreous gel inside the eye. This can be necessary when the gel is contaminated with foreign matter, such as blood. The procedure of removing the vitreous and replacing it with a substitute is called vitrectomy. Ocular tamponades are used to treat retinal detachment. When the light-sensitive retina tears or develops a hole, liquid starts to accumulate behind it. As a result the retina peels off of the back of the eye, which can lead to blindness. A tamponade material with high surface tension is injected to seal the hole temporarily until it can be closed by e.g. laser photocoagulation.[3]
Vitreous substitutes and tamponades should be transparent and biocompatible, and should have a similar refractive index as the natural gel. Commonly used materials include gases (air or fluorinated gases), saline solutions, and polymers, such as silicone oil (polysiloxanes).[4] Hydrogels are extensively investigated as vitreous substitutes, but haven’t reached clinical practice yet.
What’s next?
In this first part I gave you an overview of polymers that are already used in ophthalmic viscoelastic and drug delivery formulations. There is a lot of interesting research going on in this area, which I hope to cover more of here on the blog in future. If you liked this post and want to learn about the other applications of polymers in ophthalmology, stay tuned for part 2 dealing with Lenses, Prostheses, and other Medical Devices.
If you are using eye drops yourself, go check whether they contain any polymers and let me know in the comments! 😉
– Tina
[1] Javier Adrián Calles et al., “Polymers in Ophthalmology.” In Advanced Polymers in Medicine, edited by Francesco Puoci, 147–76. Springer International Publishing, 2015. https://doi.org/10.1007/978-3-319-12478-0_6. (Full-text: https://www.researchgate.net/publication/273631622_Polymers_in_Ophthalmology, accessed 18.10.2018).
[2] Rini Rachel Joseph & Subbu S Venkatraman, “Drug delivery to the eye: what benefits do nanocarriers offer?”, Nanomedicine (Lond.), 2017, 12(6), 683–702. https://www.futuremedicine.com/doi/10.2217/nnm-2016-0379
[3] Kamyar Vaziri et al., “Tamponade in the surgical management of retinal detachment”, Clinical Ophthalmology, 2016, 10, 471–476. https://doi.org/10.2147/OPTH.S98529
[4] Camilla Alovisi et al., “Vitreous Substitutes: Old and New Materials in Vitreoretinal Surgery”, Journal of Ophthalmology, 2017, Article ID 3172138, 6 pages. https://doi.org/10.1155/2017/3172138

[…] Hi and welcome back to part 2 of the series “Polymers in Ophthalmology”. This time, I’ll be talking about Lenses, Prostheses and other Medical Devices. If you missed part 1, you can find my post about Viscoelastic & Drug Delivery Formulations here. […]